
The new AI-driven non-invasive early detection/screening test for prostate cancer
Prostate cancer is the second most common cancer worldwide
1.4 milliondiagnoses per year
375,000deaths per year
43deaths every hour
The standard reference test PSA* discontinued due to poor accuracy
*prostate-specific antigen
Recommendations of the UK National Screening Committee and the United States Preventive Services Taskforce
Recommendations of the UK National Screening Committee and the United States Preventive Services Taskforce
High false positives3 out of 4 PSA positives do not have cancer
Significant Harm
Invasive painful biopsies: sepsis and bleeding
Unnecessary surgical prostatectomies resulting in impotence and incontinence
Inefficient
To save 1 life, PSA screening required:
1,400 screening tests
200 biopsies
48 harmful prostatectomies

Our solution
Oncologica's patented liquid biopsy test employs A.I. based cell cycle engine analysis to identify early-stage prostate cancer.A simple, accurate, life-saving test.
High accuracy (AUC 0.93 vs 0.66 for PSA)
Non-invasive: simple urine sample collected at home
No risk of permanent harm from unnecessary biopsies or surgery
Healthcare cost savings by reducing expensive invasive procedures


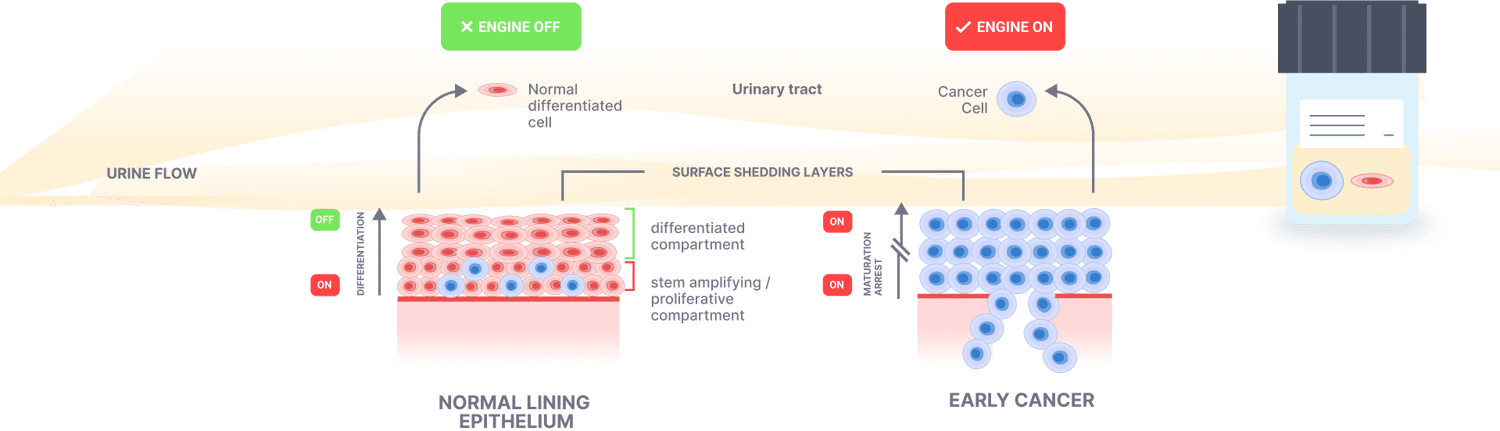
Detecting cells in urineBy analysing the maturation status and differentiation, cancer cells can be detected in the urine samples
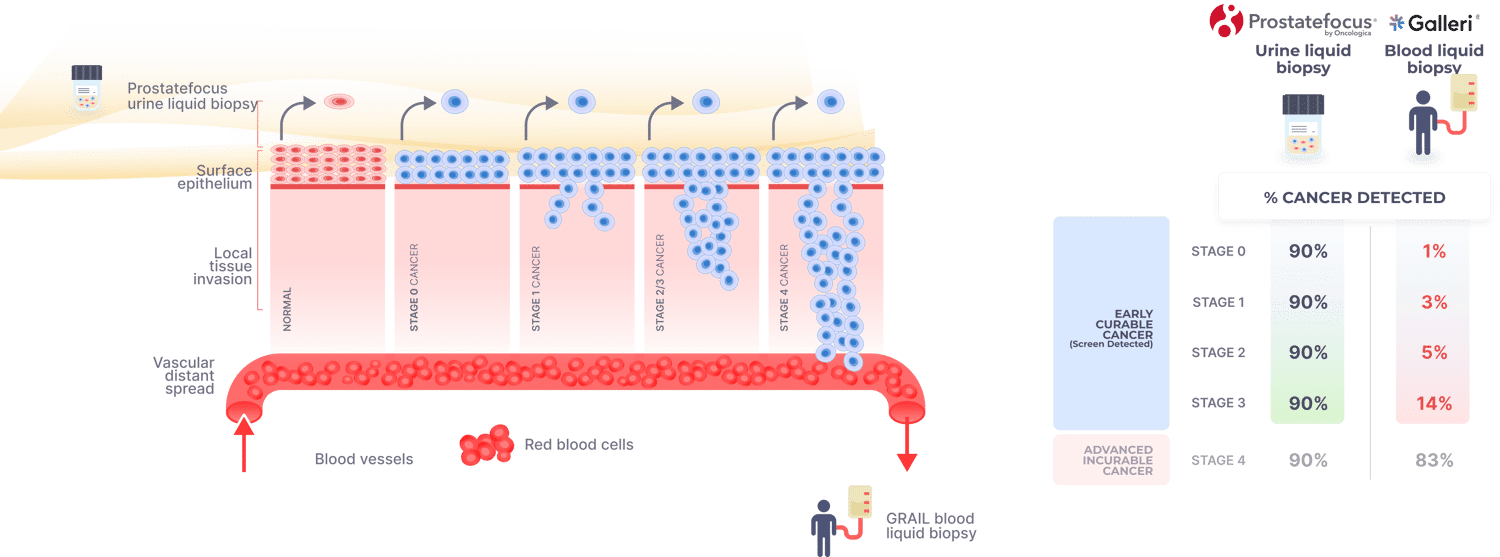
Why ProstateFocus outperforms competitors
All screening tests detect cancer cells shed from the surface epithelium, e.g. cervical PAP testDetecting cancer cells in the blood is too late, e.g. Galleri test. ProstateFocus detects shedding cells from the surface epithelium enabling detection of very early-stage disease
-1100w.png)

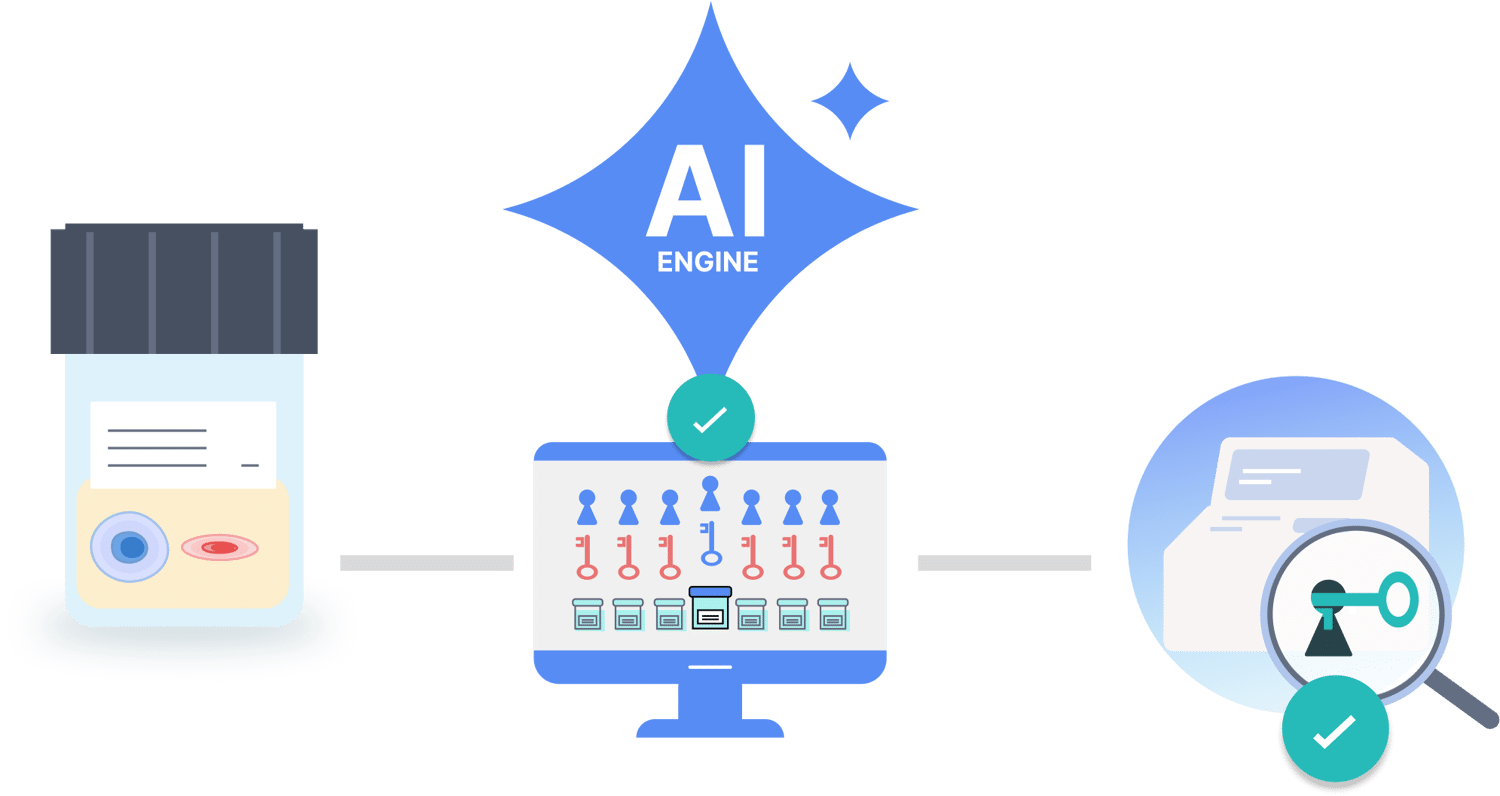
How ProstaFocus works
Three-Step AI-Driven Process
STEP 1
Biomarker SignatureCell cycle engine and linked oncogenic signaling networks analyzed from urine samples
STEP 2
AI-Driven Cancer DetectionIdentify optimal signatures for accurate cancer detection
STEP 3
AI-Driven PrognosticationAI-driven detection of prognostic prostate cancer signatures

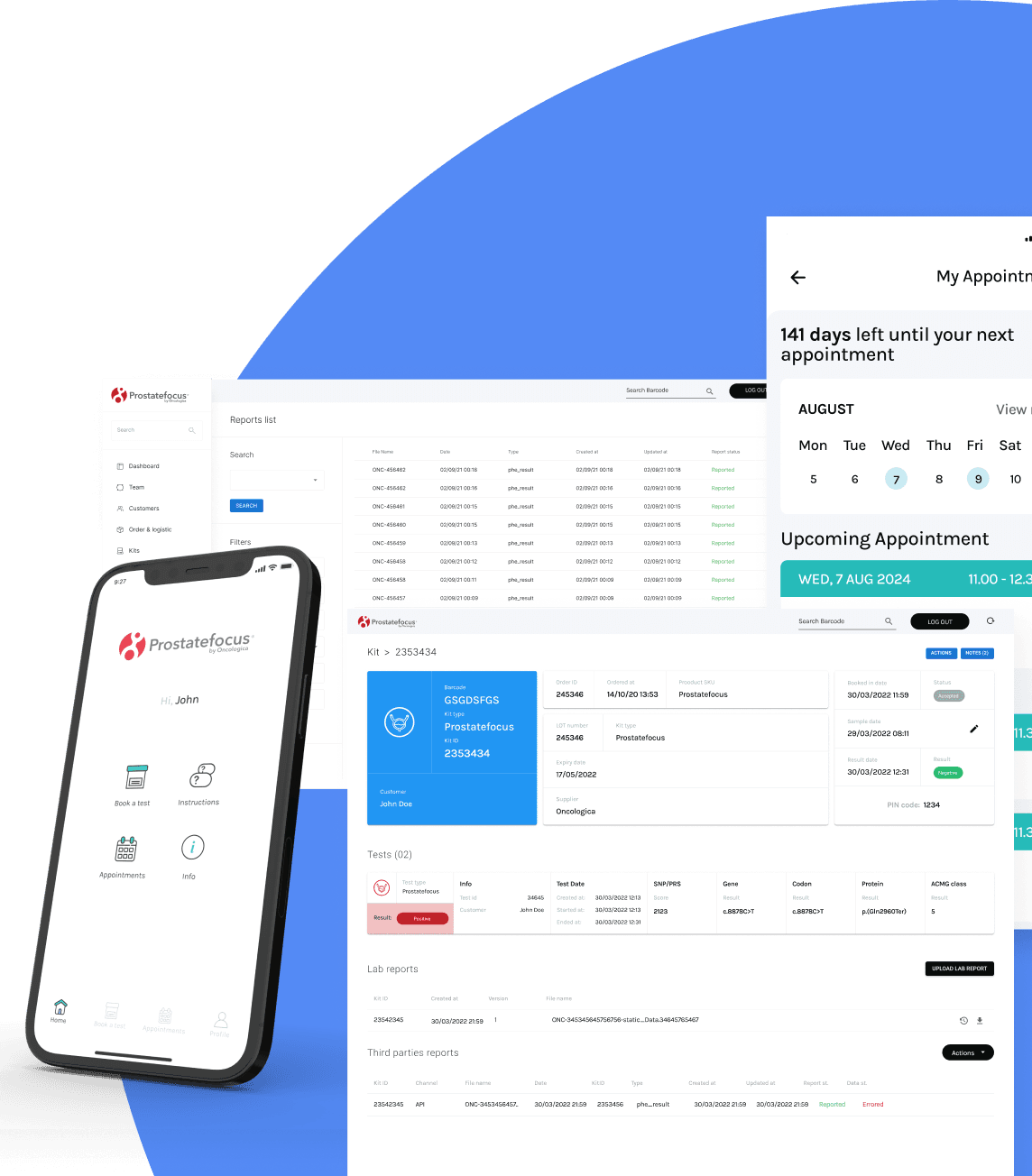
Our bespoke digital management solution
Prostatefocus comes with a proprietary and validated IT platform featuring
API interface for secure B2B connection of third-party systems and LIMS
Dedicated mobile app for patients
Built-IN CRM
Automated results reporting system
Secure encryption management
Laboratory workflow optimisation
Custom BI reporting
Reliability tested on 6milion samples
Scalability tested up to 45k concurrent users
Workload tested up to 50k/samples day
Immunofocus PD-L1 Test measures the expression levels of PD-L1 protein.
A range of cancer types have been shown to bind PD-L1 with the PD-1 of immune cells (t-cells).
This blocks the activation of the t-cell, allowing the tumour cell to evade destruction. The cancer types effected include:
A range of cancer types have been shown to bind PD-L1 with the PD-1 of immune cells (t-cells).
This blocks the activation of the t-cell, allowing the tumour cell to evade destruction. The cancer types effected include:
- Melanoma
- lung
- head and neck
- stomach
- ovarian
-min-1200w.png)
Targeting the PD-1/PD-L1 connections with inhibitors has emerged as a powerful immunotherapy against these cancer types. Inhibiting the PD-1/PD-L1 pathway with therapeutic antibodies results in restoration of immune responses and activation of
t-cells directed to kill the tumour cell.
This therapy has been shown to induce a strong clinical response in many tumour types, for example 20-40% in melanoma and 33-50% in advanced non small cell lung cancer (NSCLC).

Malfunctioning of the Mismatch Repair (MMR) pathway increases the mutational burden of specific cancers and is often involved in the cause of the malfunction, sometimes as an influential bystander and sometimes as the main driving force.
Immunofocus MMR Test analyses the Mismatch Repair genes (MLH1, MSH2, MSH6 and PMS2) to identify DNA repair defects linked to response to immunotherapy. The MMR test quickly identifies whether or not a new class of medicines will benefit you.
The MMR genetic test results provide the data needed to get access to the medicines through your oncologist or a clinical trial as quickly as possible. If the tests show that immunotherapy will benefit you, then the sooner you begin treatment the sooner you can start feeling better.
Immunofocus MMR Test analyses the Mismatch Repair genes (MLH1, MSH2, MSH6 and PMS2) to identify DNA repair defects linked to response to immunotherapy. The MMR test quickly identifies whether or not a new class of medicines will benefit you.
The MMR genetic test results provide the data needed to get access to the medicines through your oncologist or a clinical trial as quickly as possible. If the tests show that immunotherapy will benefit you, then the sooner you begin treatment the sooner you can start feeling better.
-min-200h.png)


